BIOTOP company's products are officially launched in Australian pharmacies

Australia, July 19, 2024- We are proud to announce that as a POCT rapid testing reagent sales company established in Australia, our product line has now been recognized by the Australian Medicines Agency (TGA) and officially approved
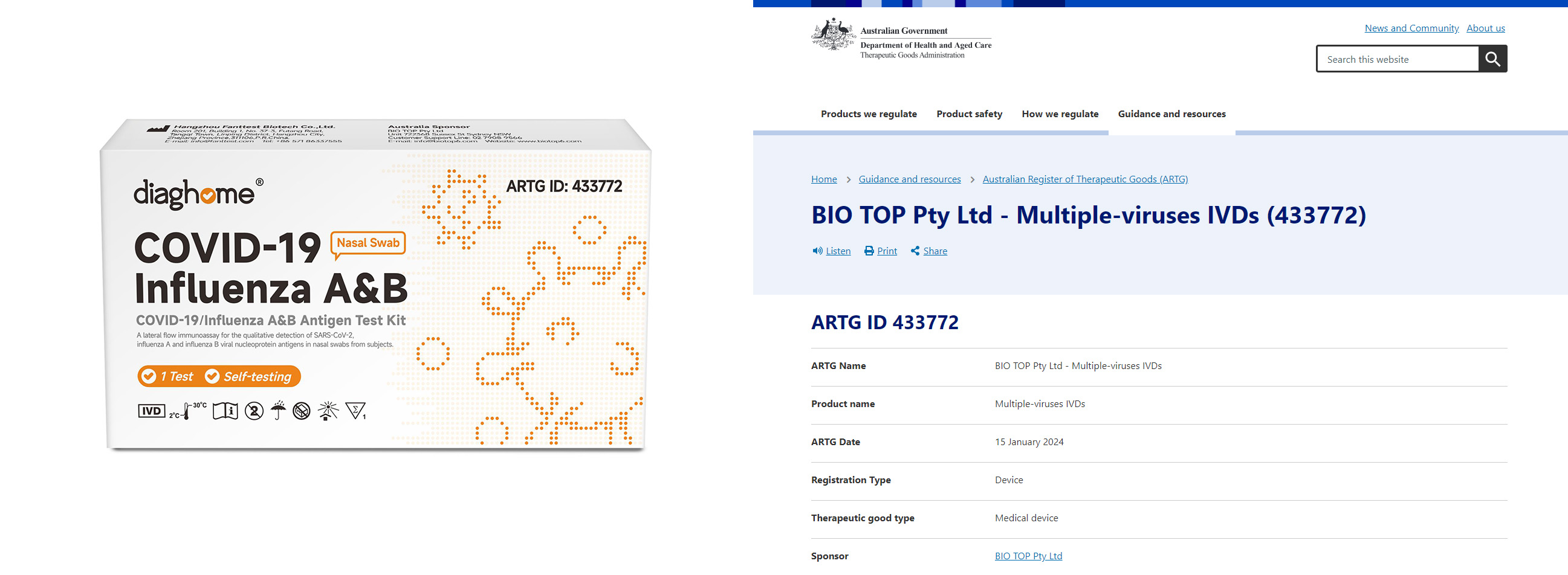
COVID-19 / Influenza A&B Antigen Test Kit:ARTG ID: 433772
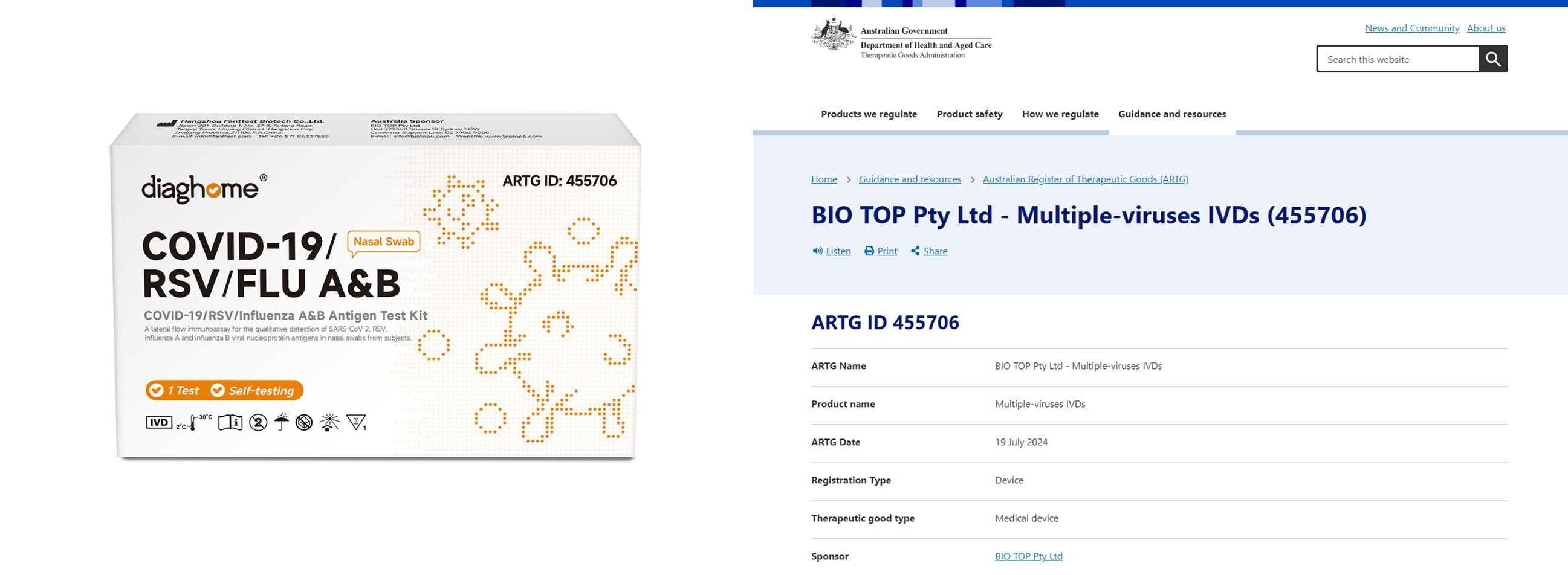
COVID-19 / RSV / Influenza A&B Antigen Test Kit:ARTG ID: 455706

COVID-19 / RSV / Influenza A&B Antigen Test Kit:ARTG ID: 455706
This important milestone marks the full launch of our product, which can be obtained in Australian pharmacies.
We have been dedicated to finding reliable products, stable air freight services, and arranging efficient customs clearance to fully meet the needs of government procurement and major pharmacies. Our products have been successfully supplied to over 30 countries worldwide, providing better solutions for efficient and rapid virus detection, personal protection, disease prevention, and epidemic control.
The newly obtained registration certificate demonstrates our commitment to continuously pursuing product quality and complying with regulatory standards. Our team will continue to be committed to providing customers with high-quality products and services to meet their needs in epidemic prevention and health protection.
We sincerely invite you to go to the Australian pharmacy to purchase our COVID-19/Influenza AB triple inspection and COVID-19/Influenza AB/Syncytial Virus 4 joint inspection products. We believe that these products will provide you with reliable, fast, and accurate virus detection solutions, so that you can understand your health status as soon as you need it.
Media contact:
If you have any media inquiries or cooperation needs, please contact our media team: info@biotop6.com
Post time: 08-13-2024